Abstract
Background Acute myeloid leukaemia (AML) is the most common acute leukaemia in adults, accounting for about 80% of cases, and accounts for 20% of childhood leukemias. Treatment of AML is similar in both high-income and low- and middle-income countries (LMICs), and utilizes an anthracycline and cytarabine backbone. Treatment-related mortality (TRM) for AML in high-income countries is significantly lower than in LMICs. Understanding the factors associated with TRM in LMICs is critical to improving AML outcomes. Here we describe the response rate to induction therapy and TRM in patients with AML treated at a tertiary cancer facility in Uganda.
Methods A retrospective study of all patients treated for AML at the Uganda Cancer Institute (UCI) in the years 2015-2020 was performed. The diagnosis of AML was based on bone marrow or peripheral blood morphology or flow cytometry. The regimen for remission induction of adult patients with AML at the UCI includes daunorubicin 60mg/m2 given intravenously on days 1-3 and cytarabine 200mg/m2 given intravenously on days 1-7 ("3+7"), while the paediatric regimen (termed "AD") consists of daunorubicin 50mg/m2 given intravenously on days 1, 3, and 5 and cytarabine 100mg/m2 given intravenously twice daily on days 1-10, followed by a second course. Consolidation therapy is given to all patients who achieve complete remission after up to 2 courses of induction. Complete remission was defined as <5% blasts in the marrow identified by morphology or flow cytometry. The study was approved by the UCI Research Ethics Committee (Ref. UCI-2020-9) and the Uganda National Council for Science and Technology (Ref. HS946ES). All patients’ information was anonymized.
Results. Medical records were reviewed for 290 patients with a diagnosis of AML, of whom 186 were further analyzed after excluding patients diagnosed with acute promyelocytic leukemia (n=33), those who did not receive induction therapy (n=66), and those who received low dose chemotherapy (n=5). The median age was 20 (IQR, 10-35) years. Patient characteristics are reported in Table 1.
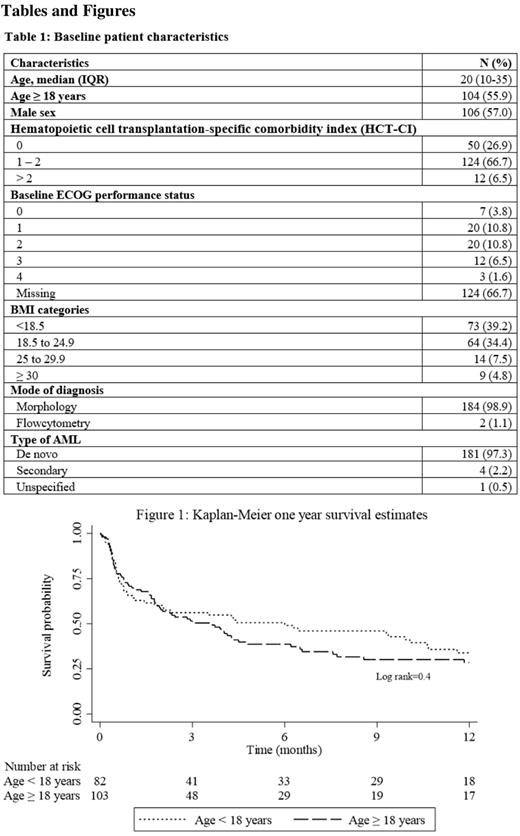
One hundred fourteen (61.3%) adult and adolescent patients received a standard "3+7” induction regimen, 65 (34.9%) children were treated with "AD,” and 7 (3.8%) patients received other regimens. Seventy four (39.8%) patients received a second course of induction. Treatment response assessment showed that 86 (46.2%) patients achieved complete remission (CR). Of these, 62 (72%) achieved CR after the first induction, with the remaining 24 (28%) after the second induction. Forty five (24.2 %) patients with evaluable response data at the end of induction did not achieve remission. A total of 55 (29.6%) patients died, all in the first induction course. The overall 30-day induction TRM rate was 31.5% (95% CI, 25.2 - 38.8) with 3-month, 6-month and one-year survival of 52.9% (95% CI, 45.3 - 60), 43.3% (95% CI, 35.8 - 50.6) and 30.8% (95% CI, 23.6 - 30.4) respectively (Figure 1). A pre-treatment HCT - CI score of >2 HR 3.37 (1.084 - 10.461), p=0.04 was associated with increased 30-day TRM.
Conclusion This study of 186 patients with AML treated at the UCI with standard induction regimens over a 5-year period demonstrates a CR rate of 46.2% and an overall 30-day induction TRM rate of 31.5%. A pre-treatment HCT-CI score of >2 was associated with increased TRM. Our study demonstrates the urgent need for interventions that can reduce TRM and improve the outcome for patients with AML in LMICs.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal